Serial Dilution Definition A method used to stepwise dilute substance into solution with constant dilution factor in each step. Application Experimental sciences include biochemistry, pharmacology, and physics as well as in homeopathy.
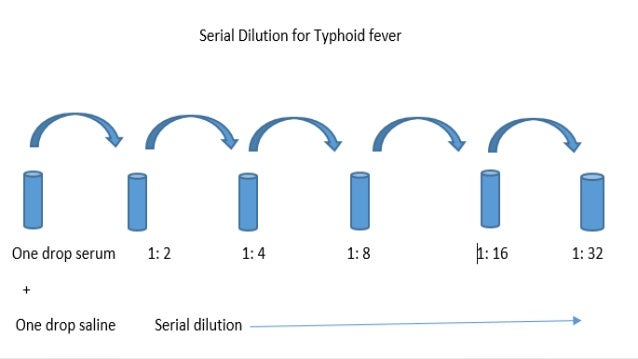

Difficulty Procedure: Medium Concept: Easy Concept Serial dilution is the stepwise dilution of a substance in solution. Usually the dilution factor at each step is constant, resulting in a geometric progression of the concentration in a logarithmic fashion.
How can the answer be improved?
Serial dilutions are used to accurately create highly diluted solutions as well as solutions for experiments resulting in concentration curves with a logarithmic scale. Serial Dilution Besides the more conventional uses described above, serial dilution may also be used to reduce the concentration of microscopic organisms or cells in a sample. A tenfold dilution for each step is called a logarithmic dilution or log-dilution and a 3.16-fold (100.5-fold) dilution is called a half-logarithmic dilution or half-log dilution. Materials • Buffer used to dissolve the sample • The sample • Multiple tubes • Pipette or graduated cylinder • Stirring rod Procedure • Shake the solution by hand or use the stirring rod to swirl the solution. Make sure the solution is uniformly mixed. • Take half of the solution out to a new tube and add equal amount of buffer into it to dilute the solution concentration to half of the original solution. • Take half of the newly made solution to another new tube and add equal amount of buffer into it to dilute the solution concentration to one quarter of the original solution.
Emergency Standby Power Systems Pdf. • Continue the process until reaching the desired concentration of the solution. Analysis Serial dilution is a process of solution preparation. Metallica Load Full Album Download Torrent.
Therefore, analysis is not necessary. References • •.